Difference between revisions of "MreD"
| Line 76: | Line 76: | ||
* '''Localization:''' | * '''Localization:''' | ||
| + | ** forms helical clusters depends {{PubMed|20566861}} | ||
| + | ** formation of helical clusters depends on the proton motive force {{PubMed|20566861}} | ||
| + | |||
=== Database entries === | === Database entries === | ||
| Line 117: | Line 120: | ||
=Labs working on this gene/protein= | =Labs working on this gene/protein= | ||
| − | |||
[[Peter Graumann]], Freiburg University, Germany [http://www.biologie.uni-freiburg.de/data/bio2/graumann/index.htm homepage] | [[Peter Graumann]], Freiburg University, Germany [http://www.biologie.uni-freiburg.de/data/bio2/graumann/index.htm homepage] | ||
| Line 123: | Line 125: | ||
=References= | =References= | ||
| − | + | ==Localization== | |
| + | <pubmed>, 20566861 </pubmed> | ||
| + | ==Other original publications== | ||
<pubmed>1400224,18156271,16101995,19643765 8459776, </pubmed> | <pubmed>1400224,18156271,16101995,19643765 8459776, </pubmed> | ||
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 13:06, 19 July 2010
- Description: MreD is a cell shape determining protein and is associated with the MreB cytoskeleton in B. subtilis and other rod shaped bacteria.
| Gene name | mreD |
| Synonyms | rodB |
| Essential | yes |
| Product | cell-shape determining protein |
| Function | cell-shape determation |
| MW, pI | 19 kDa, 7.906 |
| Gene length, protein length | 516 bp, 172 aa |
| Immediate neighbours | minC, mreC |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 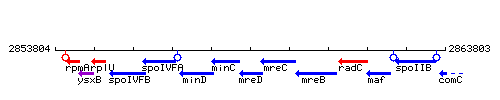
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU28010
Phenotypes of a mutant
the phenotype of mreD is similar to that of mreC. mreD is essential under normal conditions PubMed. Depletion of MreD leads to a progressive increase in the width and a decrease in the length of the cell and cells become lytic. In the depletion strain, lysis can be prevented and cell growth, but not cell shape, can be recovered by inculaion of Magnesium in the media. This shape defect is consistent with a role for mreD in cell wall synthesis during elongation and has a similar phenotype to other genes with roles in elongation.
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
MreD functions in cell wall synthesis by, together with the MreB cytoskeleton, localizing the cell wall synthetic machinery to the correct part of the cell. As a transmembrane protein MreD is thought to provide a patch on the membrane that MreB inteacts with. MreC therefore ensures that the cell wall is made in the correct way to maintain the proper shape of the cell.
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: None/ structural
- Protein family: mreD family (according to Swiss-Prot) COG2891
- Paralogous protein(s): None
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Interactions:
- Localization:
Database entries
- Structure: None
- UniProt: Q01467
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant: A conditional mutant with an inframe deletion of mreD complemented by a xylose inducible copy at an ectopic locus (strain named 4352) is avaliable from the Errington Group.
- Expression vector:
- lacZ fusion:
- GFP fusion: A functional N-terminal GFP fusion has been made where the fusion protein is the only copy of the gene in the cell: strain 3416 PubMed.
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Peter Graumann, Freiburg University, Germany homepage
Your additional remarks
References
Localization
Henrik Strahl, Leendert W Hamoen
Membrane potential is important for bacterial cell division.
Proc Natl Acad Sci U S A: 2010, 107(27);12281-6
[PubMed:20566861]
[WorldCat.org]
[DOI]
(I p)
Other original publications
Kathrin Schirner, Jeff Errington
Influence of heterologous MreB proteins on cell morphology of Bacillus subtilis.
Microbiology (Reading): 2009, 155(Pt 11);3611-3621
[PubMed:19643765]
[WorldCat.org]
[DOI]
(I p)
Alex Formstone, Rut Carballido-López, Philippe Noirot, Jeffery Errington, Dirk-Jan Scheffers
Localization and interactions of teichoic acid synthetic enzymes in Bacillus subtilis.
J Bacteriol: 2008, 190(5);1812-21
[PubMed:18156271]
[WorldCat.org]
[DOI]
(I p)
Mark Leaver, Jeff Errington
Roles for MreC and MreD proteins in helical growth of the cylindrical cell wall in Bacillus subtilis.
Mol Microbiol: 2005, 57(5);1196-209
[PubMed:16101995]
[WorldCat.org]
[DOI]
(P p)
S Lee, C W Price
The minCD locus of Bacillus subtilis lacks the minE determinant that provides topological specificity to cell division.
Mol Microbiol: 1993, 7(4);601-10
[PubMed:8459776]
[WorldCat.org]
[DOI]
(P p)
P A Levin, P S Margolis, P Setlow, R Losick, D Sun
Identification of Bacillus subtilis genes for septum placement and shape determination.
J Bacteriol: 1992, 174(21);6717-28
[PubMed:1400224]
[WorldCat.org]
[DOI]
(P p)