Difference between revisions of "HprK"
| Line 28: | Line 28: | ||
|colspan="2" | '''Genetic context''' <br/> [[Image:hprK_context.gif]] | |colspan="2" | '''Genetic context''' <br/> [[Image:hprK_context.gif]] | ||
<div align="right"> <small>This image was kindly provided by [http://genolist.pasteur.fr/SubtiList/ SubtiList]</small></div> | <div align="right"> <small>This image was kindly provided by [http://genolist.pasteur.fr/SubtiList/ SubtiList]</small></div> | ||
| + | |- | ||
| + | |colspan="2" |'''[http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=hprK_3594242_3595174_-1 Expression at a glance]'''   {{PubMed|22383849}}<br/>[[Image:hprK_expression.png|500px]] | ||
|- | |- | ||
|} | |} | ||
__TOC__ | __TOC__ | ||
| + | <br/><br/><br/><br/> | ||
| + | <br/><br/><br/><br/> | ||
| + | <br/><br/><br/><br/> | ||
| + | <br/><br/><br/><br/> | ||
| + | <br/><br/><br/><br/> | ||
| + | |||
<br/><br/><br/><br/> | <br/><br/><br/><br/> | ||
Revision as of 10:08, 23 April 2012
| Gene name | hprK |
| Synonyms | ptsK, yvoB |
| Essential | no |
| Product | HPr kinase/ phosphorylase |
| Function | carbon catabolite repression, phosphorylation of HPr and Crh proteins at Ser46 |
| Interactions involving this protein in SubtInteract: HprK | |
| Metabolic function and regulation of this protein in SubtiPathways: Central C-metabolism, Protein secretion | |
| MW, pI | 34 kDa, 4.906 |
| Gene length, protein length | 930 bp, 310 aa |
| Immediate neighbours | lgt, nagA |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 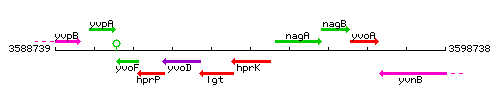
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed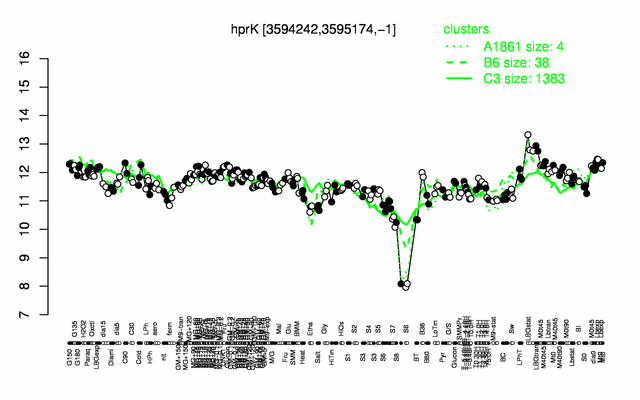
| |
Contents
Categories containing this gene/protein
protein modification, transcription factors and their control
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU35000
Phenotypes of a mutant
no carbon catabolite repression
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: ATP + HPr = ADP + P-Ser-HPr (according to Swiss-Prot)
- Protein family: HPrK/P family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- Structure: 1KKM (complex of Lactobacillus casei HprK with B. subtilis HPr-Ser-P), 1KKL (complex of Lactobacillus casei HprK with B. subtilis HPr)
- UniProt: O34483
- KEGG entry: [2]
- E.C. number:
Additional information
Expression and regulation
- Sigma factor:
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant: GP202 (spc), GP858 (aphA3), GP82 (cat), available in Stülke lab
- Expression vector:
- for expression/ purification from B. subtilis with N-terminal Strep-tag, for SPINE, in pGP380: pGP642, available in Stülke lab
- for expression/ purification of mutant HprK-G158A from B. subtilis with N-terminal Strep-tag, for SPINE, in pGP380: pGP650, available in Stülke lab
- for expression/ purification from E. coli with N-terminal His-tag, in pWH844: pGP205, available in Stülke lab
- for expression, purification of the N-terminal in E. coli with N-terminal His-tag, in pWH844: pGP218, available in Stülke lab
- GFP fusion:
- two-hybrid system:
- Antibody: available in Stülke lab
Labs working on this gene/protein
Josef Deutscher, Paris-Grignon, France
Jörg Stülke, University of Göttingen, Germany Homepage
Wolfgang Hillen, Erlangen University, Germany Homepage
Anne Galinier, University of Marseille, France
Your additional remarks
References
Reviews
Boris Görke, Jörg Stülke
Carbon catabolite repression in bacteria: many ways to make the most out of nutrients.
Nat Rev Microbiol: 2008, 6(8);613-24
[PubMed:18628769]
[WorldCat.org]
[DOI]
(I p)
Sandrine Poncet, Ivan Mijakovic, Sylvie Nessler, Virginie Gueguen-Chaignon, Vincent Chaptal, Anne Galinier, Grégory Boël, Alain Mazé, Josef Deutscher
HPr kinase/phosphorylase, a Walker motif A-containing bifunctional sensor enzyme controlling catabolite repression in Gram-positive bacteria.
Biochim Biophys Acta: 2004, 1697(1-2);123-35
[PubMed:15023355]
[WorldCat.org]
[DOI]
(P p)
Sylvie Nessler, Sonia Fieulaine, Sandrine Poncet, Anne Galinier, Josef Deutscher, Joël Janin
HPr kinase/phosphorylase, the sensor enzyme of catabolite repression in Gram-positive bacteria: structural aspects of the enzyme and the complex with its protein substrate.
J Bacteriol: 2003, 185(14);4003-10
[PubMed:12837773]
[WorldCat.org]
[DOI]
(P p)
General Analysis, Physiology
Frederik M Meyer, Matthieu Jules, Felix M P Mehne, Dominique Le Coq, Jens J Landmann, Boris Görke, Stéphane Aymerich, Jörg Stülke
Malate-mediated carbon catabolite repression in Bacillus subtilis involves the HPrK/CcpA pathway.
J Bacteriol: 2011, 193(24);6939-49
[PubMed:22001508]
[WorldCat.org]
[DOI]
(I p)
Kalpana D Singh, Matthias H Schmalisch, Jörg Stülke, Boris Görke
Carbon catabolite repression in Bacillus subtilis: quantitative analysis of repression exerted by different carbon sources.
J Bacteriol: 2008, 190(21);7275-84
[PubMed:18757537]
[WorldCat.org]
[DOI]
(I p)
Holger Ludwig, Nicole Rebhan, Hans-Matti Blencke, Matthias Merzbacher, Jörg Stülke
Control of the glycolytic gapA operon by the catabolite control protein A in Bacillus subtilis: a novel mechanism of CcpA-mediated regulation.
Mol Microbiol: 2002, 45(2);543-53
[PubMed:12123463]
[WorldCat.org]
[DOI]
(P p)
J Reizer, C Hoischen, F Titgemeyer, C Rivolta, R Rabus, J Stülke, D Karamata, M H Saier, W Hillen
A novel protein kinase that controls carbon catabolite repression in bacteria.
Mol Microbiol: 1998, 27(6);1157-69
[PubMed:9570401]
[WorldCat.org]
[DOI]
(P p)
A Galinier, M Kravanja, R Engelmann, W Hengstenberg, M C Kilhoffer, J Deutscher, J Haiech
New protein kinase and protein phosphatase families mediate signal transduction in bacterial catabolite repression.
Proc Natl Acad Sci U S A: 1998, 95(4);1823-8
[PubMed:9465101]
[WorldCat.org]
[DOI]
(P p)
Structural Analysis of HPrK
Enzymatic Properties, Mutation Analysis
HprK as a Target For Antimicrobial Compounds