Difference between revisions of "SinR"
(→Original publications) |
|||
| Line 186: | Line 186: | ||
<big>Mol Microbiol. 2011 81(6): 1459-1473. </big> | <big>Mol Microbiol. 2011 81(6): 1459-1473. </big> | ||
[http://www.ncbi.nlm.nih.gov/pubmed/21815947 PubMed:21815947] | [http://www.ncbi.nlm.nih.gov/pubmed/21815947 PubMed:21815947] | ||
| − | <pubmed>8955328,15661000,8878039,16923912,15104138,16430695,16430696,18047568,18430133,11751836,1906467,11751836,7635837,11751836, 19201793, 10547280, 15104138, 9799632 19788541 19898538 3125149 8932324 20351052 20923420 8422983 9685500 9158733 </pubmed> | + | <pubmed>8955328,15661000,8878039,16923912,15104138,16430695,16430696,18047568,18430133,11751836,1906467,11751836,7635837,11751836, 19201793, 10547280, 15104138, 9799632 19788541 19898538 3125149 8932324 20351052 20923420 8422983 9685500 9158733 23475644 </pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 08:45, 14 March 2013
- Description: transcriptional regulator of post-exponential-phase responses genes
| Gene name | sinR |
| Synonyms | sin, flaD |
| Essential | no |
| Product | transcriptional regulator of post-exponential-phase responses genes |
| Function | control of biofilm formation |
| Gene expression levels in SubtiExpress: sinR | |
| Interactions involving this protein in SubtInteract: SinR | |
| Metabolic function and regulation of this protein in SubtiPathways: Biofilm, Central C-metabolism, Protein secretion | |
| MW, pI | 12 kDa, 7.177 |
| Gene length, protein length | 333 bp, 111 aa |
| Immediate neighbours | sinI, tasA |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 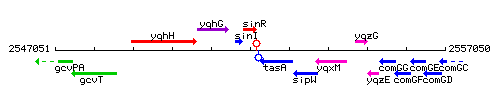
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
transcription factors and their control, transition state regulators, biofilm formation
This gene is a member of the following regulons
AbrB regulon, ScoC regulon, Spo0A regulon
The SinR regulon
The gene
Basic information
- Locus tag: BSU24610
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: transcription repressor of biofilm genes, acts as co-repressor for SlrR PubMed
- Protein family:
- Paralogous protein(s): SlrR
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- Structure:
- UniProt: P06533
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant: TMB079 sinR::spec, GP736 (tetR), available in Stülke lab
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system: B. pertussis adenylate cyclase-based bacterial two hybrid system (BACTH), available in Stülke lab
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Modelling of the SinI/SinR switch
Jennifer S Hallinan, Goksel Misirli, Anil Wipat
Evolutionary computation for the design of a stochastic switch for synthetic genetic circuits.
Annu Int Conf IEEE Eng Med Biol Soc: 2010, 2010;768-74
[PubMed:21095906]
[WorldCat.org]
[DOI]
(P p)
Original publications
Additonal publications: PubMed
Joseph A Newman, Cecilia Rodrigues, Richard J Lewis
Molecular basis of the activity of SinR protein, the master regulator of biofilm formation in Bacillus subtilis.
J Biol Chem: 2013, 288(15);10766-78
[PubMed:23430750]
[WorldCat.org]
[DOI]
(I p)
Ying Lei, Taku Oshima, Naotake Ogasawara, Shu Ishikawa
Functional analysis of the protein Veg, which stimulates biofilm formation in Bacillus subtilis.
J Bacteriol: 2013, 195(8);1697-705
[PubMed:23378512]
[WorldCat.org]
[DOI]
(I p)
Yunrong Chai, Pascale B Beauregard, Hera Vlamakis, Richard Losick, Roberto Kolter
Galactose metabolism plays a crucial role in biofilm formation by Bacillus subtilis.
mBio: 2012, 3(4);e00184-12
[PubMed:22893383]
[WorldCat.org]
[DOI]
(I e)
Diethmaier C, Pietack N, Gunka K, Wrede C, Lehnik-Habrink M, Herzberg C, Hübner S, Stülke J A Novel Factor Controlling Bistability in Bacillus subtilis: The YmdB Protein Affects Flagellin Expression and Biofilm Formation. J Bacteriol.: 2011, 193(21):5997-6007. PubMed:21856853
Lehnik-Habrink M, Schaffer M, Mäder U, Diethmaier C, Herzberg C, Stülke J RNA processing in Bacillus subtilis: identification of targets of the essential RNase Y. Mol Microbiol. 2011 81(6): 1459-1473. PubMed:21815947