Difference between revisions of "QueA"
| Line 119: | Line 119: | ||
* '''Additional information:''' | * '''Additional information:''' | ||
| + | ** number of protein molecules per cell (minimal medium with glucose and ammonium): 154 {{PubMed|24696501}} | ||
| + | ** number of protein molecules per cell (complex medium with amino acids, without glucose): 395 {{PubMed|24696501}} | ||
=Biological materials = | =Biological materials = | ||
Revision as of 09:40, 17 April 2014
- Description: S-adenosylmethionine tRNA ribosyltransferase
| Gene name | queA |
| Synonyms | |
| Essential | no |
| Product | S-adenosylmethionine tRNA ribosyltransferase |
| Function | tRNA modification |
| Gene expression levels in SubtiExpress: queA | |
| MW, pI | 38 kDa, 5.047 |
| Gene length, protein length | 1026 bp, 342 aa |
| Immediate neighbours | tgt, yrzS |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 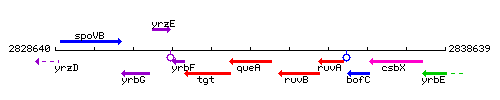
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU27720
Phenotypes of a mutant
Database entries
- BsubCyc: BSU27720
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: S-adenosylmethionine + 7-aminomethyl-7-deazaguanosine = methionine + adenine + epoxyqueuosine (according to Swiss-Prot)
- Protein family: queA family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- BsubCyc: BSU27720
- Structure: 1YY3
- UniProt: O32054
- KEGG entry: [2]
- E.C. number:
Additional information
Expression and regulation
- Operon:
- Sigma factor:
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Clemens Grimm, Ralf Ficner, Tanja Sgraja, Peter Haebel, Gerhard Klebe, Klaus Reuter
Crystal structure of Bacillus subtilis S-adenosylmethionine:tRNA ribosyltransferase-isomerase.
Biochem Biophys Res Commun: 2006, 351(3);695-701
[PubMed:17083917]
[WorldCat.org]
[DOI]
(P p)
C Grimm, G Klebe, R Ficner, K Reuter
Screening orthologs as an important variable in crystallization: preliminary X-ray diffraction studies of the tRNA-modifying enzyme S-adenosyl-methionine:tRNA ribosyl transferase/isomerase.
Acta Crystallogr D Biol Crystallogr: 2000, 56(Pt 4);484-8
[PubMed:10739928]
[WorldCat.org]
[DOI]
(P p)