Difference between revisions of "Nos"
| Line 139: | Line 139: | ||
=References= | =References= | ||
| − | <pubmed>12220171,11856757,20006999 , 21599925 22119809 23943262 21310962,21921039 25194416</pubmed> | + | ==Reviews== |
| + | <pubmed>9818193</pubmed> | ||
| + | ==Original publications== | ||
| + | <pubmed>12220171,11856757,20006999 , 21599925 22119809 23943262 21310962,21921039 25194416 25826316</pubmed> | ||
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 11:36, 1 April 2015
- Description: nitric-oxide synthase
| Gene name | nos |
| Synonyms | yflM |
| Essential | no |
| Product | nitric-oxide synthase |
| Function | production of nitric oxide |
| Gene expression levels in SubtiExpress: nos | |
| Interactions involving this protein in SubtInteract: Nos | |
| MW, pI | 38 kDa, 5.528 |
| Gene length, protein length | 1008 bp, 336 aa |
| Immediate neighbours | yflN, yflL |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed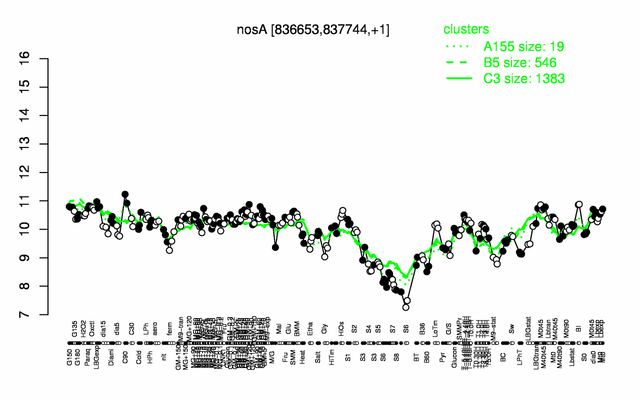
| |
Contents
Categories containing this gene/protein
miscellaneous metabolic pathways, biosynthesis of antibacterial compounds
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU07630
Phenotypes of a mutant
Database entries
- BsubCyc: BSU07630
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- NADPH-dependent oxidation of l-arginine into l-citrulline PubMed
- Protein family: Bacterial NOS oxygenase subfamily (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Modification:
- Effectors of protein activity:
Database entries
- BsubCyc: BSU07630
- UniProt: O34453
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Sigma factor:
- Regulation:
- Regulatory mechanism:
- Additional information: Note that the gene yflL is located between nos and yflK, but on the opposite strand.
- number of protein molecules per cell (minimal medium with glucose and ammonium): 18 PubMed
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
Original publications
Andreas Mielcarek, Bastian Blauenburg, Marcus Miethke, Mohamed A Marahiel
Molecular insights into frataxin-mediated iron supply for heme biosynthesis in Bacillus subtilis.
PLoS One: 2015, 10(3);e0122538
[PubMed:25826316]
[WorldCat.org]
[DOI]
(I e)
Jeffrey K Holden, Nathan Lim, Thomas L Poulos
Identification of redox partners and development of a novel chimeric bacterial nitric oxide synthase for structure activity analyses.
J Biol Chem: 2014, 289(42);29437-45
[PubMed:25194416]
[WorldCat.org]
[DOI]
(I p)
Jérôme Santolini, Amandine Maréchal, Alain Boussac, Pierre Dorlet
EPR characterisation of the ferrous nitrosyl complex formed within the oxygenase domain of NO synthase.
Chembiochem: 2013, 14(14);1852-7
[PubMed:23943262]
[WorldCat.org]
[DOI]
(I p)
Isabelle Salard-Arnaud, Dennis Stuehr, Jean-Luc Boucher, Daniel Mansuy
Spectroscopic, catalytic and binding properties of Bacillus subtilis NO synthase-like protein: comparison with other bacterial and mammalian NO synthases.
J Inorg Biochem: 2012, 106(1);164-71
[PubMed:22119809]
[WorldCat.org]
[DOI]
(I p)
Luciana Hannibal, Ramasamy Somasundaram, Jesús Tejero, Adjele Wilson, Dennis J Stuehr
Influence of heme-thiolate in shaping the catalytic properties of a bacterial nitric-oxide synthase.
J Biol Chem: 2011, 286(45);39224-35
[PubMed:21921039]
[WorldCat.org]
[DOI]
(I p)
Frank Schreiber, Martin Beutler, Dennis Enning, María Lamprecht-Grandio, Olga Zafra, José Eduardo González-Pastor, Dirk de Beer
The role of nitric-oxide-synthase-derived nitric oxide in multicellular traits of Bacillus subtilis 3610: biofilm formation, swarming, and dispersal.
BMC Microbiol: 2011, 11;111
[PubMed:21599925]
[WorldCat.org]
[DOI]
(I e)
Albane Brunel, Adjélé Wilson, Laura Henry, Pierre Dorlet, Jérôme Santolini
The proximal hydrogen bond network modulates Bacillus subtilis nitric-oxide synthase electronic and structural properties.
J Biol Chem: 2011, 286(14);11997-2005
[PubMed:21310962]
[WorldCat.org]
[DOI]
(I p)
Zhi-Qiang Wang, Chin-Chuan Wei, Dennis J Stuehr
How does a valine residue that modulates heme-NO binding kinetics in inducible NO synthase regulate enzyme catalysis?
J Inorg Biochem: 2010, 104(3);349-56
[PubMed:20006999]
[WorldCat.org]
[DOI]
(I p)
Kartikeya Pant, Alexandrine M Bilwes, Subrata Adak, Dennis J Stuehr, Brian R Crane
Structure of a nitric oxide synthase heme protein from Bacillus subtilis.
Biochemistry: 2002, 41(37);11071-9
[PubMed:12220171]
[WorldCat.org]
[DOI]
(P p)
Subrata Adak, Kulwant S Aulak, Dennis J Stuehr
Direct evidence for nitric oxide production by a nitric-oxide synthase-like protein from Bacillus subtilis.
J Biol Chem: 2002, 277(18);16167-71
[PubMed:11856757]
[WorldCat.org]
[DOI]
(P p)