Difference between revisions of "Abn2"
(→References) |
(→References) |
||
| Line 123: | Line 123: | ||
=References= | =References= | ||
| − | <pubmed>18607095,,18408032,18957862 12850135 18408032 | + | <pubmed>18607095,,18408032,18957862 12850135 18408032 </pubmed> |
| − | + | '''Additional references:''' {{PubMed|20883454}} | |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 10:02, 6 October 2010
- Description: endo-1,5-alpha-L-arabinosidase
| Gene name | abn2 |
| Synonyms | yxiA |
| Essential | no |
| Product | endo-1,5-alpha-L-arabinosidase |
| Function | arabinan degradation |
| MW, pI | 52 kDa, 7.371 |
| Gene length, protein length | 1407 bp, 469 aa |
| Immediate neighbours | yxiB, hutP |
| Gene sequence (+200bp) | Protein sequence |
| Caution: The sequence for this gene in SubtiList contains errors | |
Genetic context 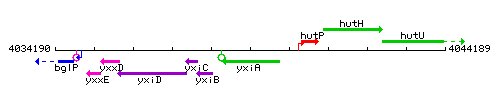
| |
Contents
The gene
Basic information
- Locus tag:
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: glycosyl hydrolase 43 family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information: With linear-alpha-1,5-l-arabinan as the preferred substrate, the enzyme exhibited an apparent K(m) of 2.0 mg ml(-1) and V(max) of 0.25 mmol min(-1) mg(-1) at pH 7.0 and 50°C. PubMed
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Interactions:
- Localization: extracellular (signal peptide) PubMed
Database entries
- Structure:
- UniProt: P42293
- KEGG entry: [3]
- E.C. number: 3.2.1.99
Additional information
Expression and regulation
- Operon: abn2 PubMed
- Regulation: repressed by glucose (4.3-fold) (CcpA) PubMed, expression is stimulated by arabinose and pectin and repressed by glucose PubMed
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Isabel de Sa-Nogueira, Lisboa, Portugal homepage
Your additional remarks
References
Additional references: PubMed