Difference between revisions of "UgtP"
(→Original Publications) |
|||
| Line 155: | Line 155: | ||
==Original Publications== | ==Original Publications== | ||
'''Additional publications:''' {{PubMed|22931116}} | '''Additional publications:''' {{PubMed|22931116}} | ||
| − | <pubmed>9244290,18820022,17662947,9720862 22362028 15640167 | + | <pubmed>9244290,18820022,17662947,9720862 22362028 15640167 </pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 09:31, 30 October 2012
- Description: UDP-glucose diacylglycerol glucosyltransferase, growth-rate dependent inhibitor of cell division
| Gene name | ugtP |
| Synonyms | ypfP |
| Essential | no |
| Product | UDP-glucose diacylglycerol glucosyltransferase |
| Function | synthesis of glycolipids and anchoring of lipoteichoic acid, inhibition of FtsZ assembly |
| Gene expression levels in SubtiExpress: ugtP | |
| Interactions involving this protein in SubtInteract: UgtP | |
| Metabolic function and regulation of this protein in SubtiPathways: Lipid synthesis | |
| MW, pI | 43 kDa, 8.398 |
| Gene length, protein length | 1146 bp, 382 aa |
| Immediate neighbours | metA, cspD |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 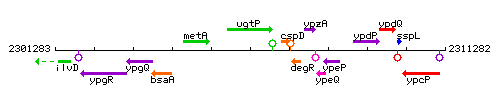
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
cell division, lipid metabolism/ other, cell envelope stress proteins (controlled by SigM, V, W, X, Y), membrane proteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU21920
Phenotypes of a mutant
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- UDP-glucose + 1,2-diacylglycerol = UDP + 1,2-diacyl-3-(O-beta-D-glucopyranosyl)-sn-glycerol (according to Swiss-Prot)
- the interaction with FtsZ results in inhibition of cell division and an increase of cell size PubMed
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization:
- membrane-bound protein, self-assembles into tightly wound spirals in vitro PubMed
- under nutrient rich conditions (increased concentration of UDP-Glc): throughout the cell, concentrated at the cell poles and/or the cytokinetic ring, interaction with FtsZ PubMed
- under nutrient poor conditions: forms punctate foci (oligomers), no interaction with FtsZ PubMed
Database entries
- Structure:
- UniProt: P54166
- KEGG entry: [2]
- E.C. number:
Additional information
Expression and regulation
- Operon:
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
An-Chun Chien, Norbert S Hill, Petra Anne Levin
Cell size control in bacteria.
Curr Biol: 2012, 22(9);R340-9
[PubMed:22575476]
[WorldCat.org]
[DOI]
(I p)
David W Adams, Jeff Errington
Bacterial cell division: assembly, maintenance and disassembly of the Z ring.
Nat Rev Microbiol: 2009, 7(9);642-53
[PubMed:19680248]
[WorldCat.org]
[DOI]
(I p)
Daisuke Shiomi, William Margolin
A sweet sensor for size-conscious bacteria.
Cell: 2007, 130(2);216-8
[PubMed:17662935]
[WorldCat.org]
[DOI]
(P p)
Original Publications
Additional publications: PubMed