Difference between revisions of "SsbA"
(→References) |
|||
| Line 144: | Line 144: | ||
=References= | =References= | ||
'''Additional publications:''' {{PubMed|21859751,21170359,21958350}} | '''Additional publications:''' {{PubMed|21859751,21170359,21958350}} | ||
| − | <pubmed>17853894,19730681 11948146,16479537,11948165 16549871 20122408 14762004 20581116 22054219</pubmed> | + | <pubmed>17853894,19730681 11948146,16479537,11948165 16549871 20122408 14762004 20581116 22054219 22373918 </pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 17:52, 5 March 2012
- Description: single-strand DNA-binding protein, part of the replisome
| Gene name | ssbA |
| Synonyms | ssb |
| Essential | yes PubMed |
| Product | single-strand DNA-binding protein |
| Function | DNA replication, DNA repair/ recombination |
| Interactions involving this protein in SubtInteract: SsbA | |
| MW, pI | 18 kDa, 4.822 |
| Gene length, protein length | 516 bp, 172 aa |
| Immediate neighbours | rpsR, rpsF |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 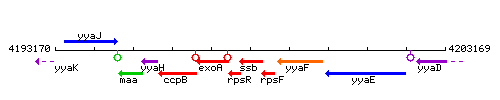
This image was kindly provided by SubtiList
| |
Contents
Categories containing this gene/protein
DNA replication, DNA repair/ recombination, essential genes
This gene is a member of the following regulons
ComK regulon, stringent response
The gene
Basic information
- Locus tag: BSU40900
Phenotypes of a mutant
essential PubMed
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family:
- Paralogous protein(s): SsbB
Extended information on the protein
- Kinetic information:
- Domains:
- Cofactor(s):
- Effectors of protein activity: Phosphorylation of Ssb increases binding to single-stranded DNA in vitro almost 200-fold PubMed
Database entries
- Structure:
- UniProt: P37455
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Sigma factor:
- Regulation:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Additional publications: PubMed
Tribhuwan Yadav, Begoña Carrasco, Angela R Myers, Nicholas P George, James L Keck, Juan C Alonso
Genetic recombination in Bacillus subtilis: a division of labor between two single-strand DNA-binding proteins.
Nucleic Acids Res: 2012, 40(12);5546-59
[PubMed:22373918]
[WorldCat.org]
[DOI]
(I p)
Wei Zhang, Xiujuan Lü, Wenke Zhang, Jiacong Shen
EMSA and single-molecule force spectroscopy study of interactions between Bacillus subtilis single-stranded DNA-binding protein and single-stranded DNA.
Langmuir: 2011, 27(24);15008-15
[PubMed:22054219]
[WorldCat.org]
[DOI]
(I p)
Candela Manfredi, Yuki Suzuki, Tribhuwan Yadav, Kunio Takeyasu, Juan C Alonso
RecO-mediated DNA homology search and annealing is facilitated by SsbA.
Nucleic Acids Res: 2010, 38(20);6920-9
[PubMed:20581116]
[WorldCat.org]
[DOI]
(I p)
Glenn M Sanders, H Garry Dallmann, Charles S McHenry
Reconstitution of the B. subtilis replisome with 13 proteins including two distinct replicases.
Mol Cell: 2010, 37(2);273-81
[PubMed:20122408]
[WorldCat.org]
[DOI]
(I p)
Dawit Kidane, Begoña Carrasco, Candela Manfredi, Katharina Rothmaier, Silvia Ayora, Serkalem Tadesse, Juan C Alonso, Peter L Graumann
Evidence for different pathways during horizontal gene transfer in competent Bacillus subtilis cells.
PLoS Genet: 2009, 5(9);e1000630
[PubMed:19730681]
[WorldCat.org]
[DOI]
(I p)
François Lecointe, Céline Sérèna, Marion Velten, Audrey Costes, Stephen McGovern, Jean-Christophe Meile, Jeffrey Errington, S Dusko Ehrlich, Philippe Noirot, Patrice Polard
Anticipating chromosomal replication fork arrest: SSB targets repair DNA helicases to active forks.
EMBO J: 2007, 26(19);4239-51
[PubMed:17853894]
[WorldCat.org]
[DOI]
(P p)
Ivan Mijakovic, Dina Petranovic, Boris Macek, Tina Cepo, Matthias Mann, Julian Davies, Peter R Jensen, Dusica Vujaklija
Bacterial single-stranded DNA-binding proteins are phosphorylated on tyrosine.
Nucleic Acids Res: 2006, 34(5);1588-96
[PubMed:16549871]
[WorldCat.org]
[DOI]
(I e)
Jean-Christophe Meile, Ling Juan Wu, S Dusko Ehrlich, Jeff Errington, Philippe Noirot
Systematic localisation of proteins fused to the green fluorescent protein in Bacillus subtilis: identification of new proteins at the DNA replication factory.
Proteomics: 2006, 6(7);2135-46
[PubMed:16479537]
[WorldCat.org]
[DOI]
(P p)
Cordula Lindner, Reindert Nijland, Mariska van Hartskamp, Sierd Bron, Leendert W Hamoen, Oscar P Kuipers
Differential expression of two paralogous genes of Bacillus subtilis encoding single-stranded DNA binding protein.
J Bacteriol: 2004, 186(4);1097-105
[PubMed:14762004]
[WorldCat.org]
[DOI]
(P p)
Christine Eymann, Georg Homuth, Christian Scharf, Michael Hecker
Bacillus subtilis functional genomics: global characterization of the stringent response by proteome and transcriptome analysis.
J Bacteriol: 2002, 184(9);2500-20
[PubMed:11948165]
[WorldCat.org]
[DOI]
(P p)
Mitsuo Ogura, Hirotake Yamaguchi, Kazuo Kobayashi, Naotake Ogasawara, Yasutaro Fujita, Teruo Tanaka
Whole-genome analysis of genes regulated by the Bacillus subtilis competence transcription factor ComK.
J Bacteriol: 2002, 184(9);2344-51
[PubMed:11948146]
[WorldCat.org]
[DOI]
(P p)