Difference between revisions of "AccB"
(→Original Publications) |
(→Biological materials) |
||
| Line 131: | Line 131: | ||
* '''Expression vector:''' | * '''Expression vector:''' | ||
| + | ** GP1477 (chromosomal ''accB''-Strep fusion, ''aphA''3), purification from ''B. subtilis'', for [[SPINE]], available in [[Jörg Stülke]]'s lab''' | ||
* '''lacZ fusion:''' | * '''lacZ fusion:''' | ||
| Line 136: | Line 137: | ||
* '''GFP fusion:''' | * '''GFP fusion:''' | ||
| − | * '''two-hybrid system:''' | + | * '''two-hybrid system:''' ''B. pertussis'' adenylate cyclase-based bacterial two hybrid system ([[BACTH]]), available in [[Jörg Stülke]]'s lab |
| + | |||
* '''Antibody:''' | * '''Antibody:''' | ||
Revision as of 09:54, 6 March 2015
- Description: acetyl-CoA carboxylase (biotin carboxyl carrier subunit)
| Gene name | accB |
| Synonyms | fabE, yqhW |
| Essential | yes PubMed |
| Product | acetyl-CoA carboxylase (biotin carboxyl carrier subunit) |
| Function | production of malonyl-CoA, the substrate for fatty acid biosynthesis |
| Gene expression levels in SubtiExpress: accB | |
| Interactions involving this protein in SubtInteract: AccB | |
| Metabolic function and regulation of this protein in SubtiPathways: accB | |
| MW, pI | 17 kDa, 4.394 |
| Gene length, protein length | 477 bp, 159 aa |
| Immediate neighbours | accC, spoIIIAH |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 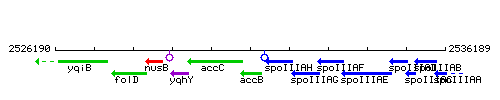
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed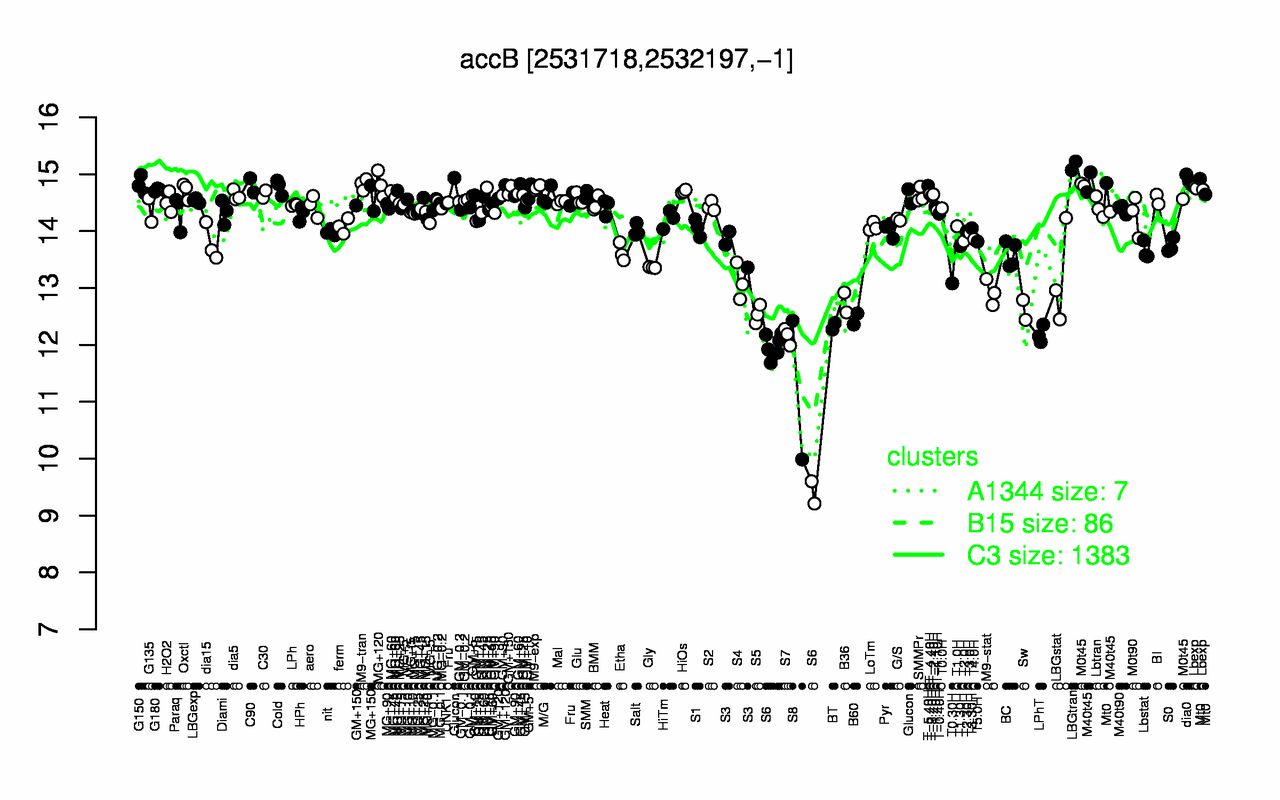
| |
Contents
Categories containing this gene/protein
biosynthesis of lipids, essential genes
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU24350
Phenotypes of a mutant
essential PubMed
Database entries
- BsubCyc: BSU24350
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactors: biotin
- Effectors of protein activity:
Database entries
- BsubCyc: BSU24350
- UniProt: P49786
- KEGG entry: [3]
- E.C. number: 6.4.1.2
Additional information
AccB binds to StrepTactin, and may be co-purified when purifying Strep-tagged proteins by SPINE.
Expression and regulation
- Sigma factor:
- Regulation:
- Regulatory mechanism:
- Additional information:
- number of protein molecules per cell (minimal medium with glucose and ammonium, exponential phase): 3355 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, early stationary phase after glucose exhaustion): 889 PubMed
- number of protein molecules per cell (minimal medium with glucose and ammonium, late stationary phase after glucose exhaustion): 28 PubMed
Biological materials
- Mutant:
- Expression vector:
- GP1477 (chromosomal accB-Strep fusion, aphA3), purification from B. subtilis, for SPINE, available in Jörg Stülke's lab
- lacZ fusion:
- GFP fusion:
- two-hybrid system: B. pertussis adenylate cyclase-based bacterial two hybrid system (BACTH), available in Jörg Stülke's lab
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Reviews
L Tong
Acetyl-coenzyme A carboxylase: crucial metabolic enzyme and attractive target for drug discovery.
Cell Mol Life Sci: 2005, 62(16);1784-803
[PubMed:15968460]
[WorldCat.org]
[DOI]
(P p)
Stephen W White, Jie Zheng, Yong-Mei Zhang, Rock
The structural biology of type II fatty acid biosynthesis.
Annu Rev Biochem: 2005, 74;791-831
[PubMed:15952903]
[WorldCat.org]
[DOI]
(P p)
John E Cronan, Grover L Waldrop
Multi-subunit acetyl-CoA carboxylases.
Prog Lipid Res: 2002, 41(5);407-35
[PubMed:12121720]
[WorldCat.org]
[DOI]
(P p)
Original Publications
F K Athappilly, W A Hendrickson
Structure of the biotinyl domain of acetyl-coenzyme A carboxylase determined by MAD phasing.
Structure: 1995, 3(12);1407-19
[PubMed:8747466]
[WorldCat.org]
[DOI]
(P p)
P Marini, S J Li, D Gardiol, J E Cronan, D de Mendoza
The genes encoding the biotin carboxyl carrier protein and biotin carboxylase subunits of Bacillus subtilis acetyl coenzyme A carboxylase, the first enzyme of fatty acid synthesis.
J Bacteriol: 1995, 177(23);7003-6
[PubMed:7592499]
[WorldCat.org]
[DOI]
(P p)