Spo0A
- Description: phosphorelay regulator, initiation of sporulation, coordinates DNA replication and initiation of sporulation by binding to sites close to the oriC
| Gene name | spo0A |
| Synonyms | spo0C, spo0G, spoIIL, sof-1 |
| Essential | no |
| Product | phosphorelay response regulator |
| Function | initiation of sporulation |
| Gene expression levels in SubtiExpress: spo0A | |
| Interactions involving this protein in SubtInteract: Spo0A | |
| Metabolic function and regulation of this protein in SubtiPathways: Biofilm, Nucleotides (regulation), Ammonium/ glutamate, Central C-metabolism,Sugar catabolism, Phosphorelay, Stress, tRNA charging,Lipid synthesis, Protein secretion | |
| MW, pI | 29 kDa, 5.989 |
| Gene length, protein length | 801 bp, 267 aa |
| Immediate neighbours | yqiG, spoIVB |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 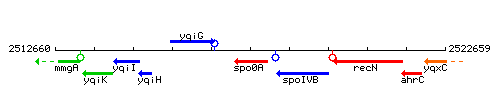
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed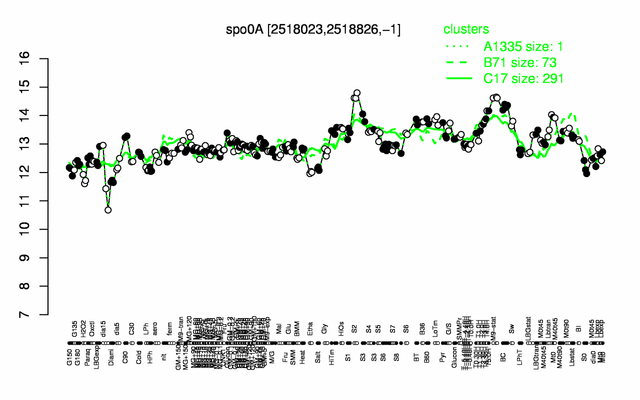
| |
Contents
Categories containing this gene/protein
transcription factors and their control, phosphorelay, biofilm formation, phosphoproteins
This gene is a member of the following regulons
The Spo0A regulon
The gene
Basic information
- Locus tag: BSU24220
Phenotypes of a mutant
- inactivation of spo0A restores beta-lactam resistance in a sigM mutant PubMed
- altered cell death pattern in colonies PubMed
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- activation of gene expression at the onset of sporulation (Spo0A regulon)
- coordination between DNA replication and initiation of sporulation by binding to sites close to the oriC (this limits re-initiation of replication) PubMed
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification: receives phosphorylation from Spo0B, dephosphorylation by Spo0E, direct phosphorylation by KinC upon potassium leakage PubMed
- Cofactor(s):
- Effectors of protein activity:
Database entries
- Structure: 1QMP (with phosphorylated aspartate, Geobacillus stearothermophilus), 1FC3 (trans-activation domain, Geobacillus stearothermophilus), 1LQ1 (complex with DNA)
- UniProt: P06534
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Operon: spo0A PubMed
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
- Tony Wilkinson, York University, U.K. homepage
- Imrich Barak, Slovak Academy of Science, Bratislava, Slovakia homepage
- Charles Moran, Emory University, NC, USA homepage
Your additional remarks
References
Reviews
The Spo0A regulon
Other original Publications
Additional publications: PubMed
TTTTT
ÜÜÜ
Mirjam Boonstra, Imke G de Jong, Graham Scholefield, Heath Murray, Oscar P Kuipers, Jan-Willem Veening
Spo0A regulates chromosome copy number during sporulation by directly binding to the origin of replication in Bacillus subtilis.
Mol Microbiol: 2013, 87(4);925-38
[PubMed:23301687]
[WorldCat.org]
[DOI]
(I p)
Munehiro Asally, Mark Kittisopikul, Pau Rué, Yingjie Du, Zhenxing Hu, Tolga Çağatay, Andra B Robinson, Hongbing Lu, Jordi Garcia-Ojalvo, Gürol M Süel
Localized cell death focuses mechanical forces during 3D patterning in a biofilm.
Proc Natl Acad Sci U S A: 2012, 109(46);18891-6
[PubMed:23012477]
[WorldCat.org]
[DOI]
(I p)
Fordyce A Davidson, Chung Seon-Yi, Nicola R Stanley-Wall
Selective heterogeneity in exoprotease production by Bacillus subtilis.
PLoS One: 2012, 7(6);e38574
[PubMed:22745669]
[WorldCat.org]
[DOI]
(I p)