CcpA
- Description: Carbon catabolite control protein A, involved in glucose regulation of many genes; represses catabolic genes and activates genes involved in excretion of excess carbon
| Gene name | ccpA |
| Synonyms | graR, alsA, amyR |
| Essential | no |
| Product | transcriptional regulator (LacI family) |
| Function | mediates carbon catabolite repression (CCR) |
| Gene expression levels in SubtiExpress: ccpA | |
| Interactions involving this protein in SubtInteract: CcpA | |
| Metabolic function and regulation of this protein in SubtiPathways: Nucleoside catabolism, Nucleotides (regulation), Ile, Leu, Val, His, Coenzyme A, Central C-metabolism | |
| MW, pI | 36,8 kDa, 5.06 |
| Gene length, protein length | 1002 bp, 334 amino acids |
| Immediate neighbours | motP, aroA |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 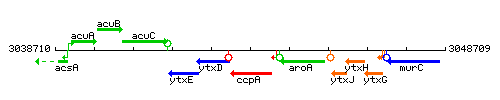
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
- 1 Categories containing this gene/protein
- 2 This gene is a member of the following regulons
- 3 The CcpA regulon
- 4 The gene
- 5 The protein
- 6 Expression and regulation
- 7 Biological materials
- 8 Labs working on this gene/protein
- 9 Your additional remarks
- 10 References
- 10.1 Reviews
- 10.2 General and physiological studies
- 10.3 Global analyses (proteome, transcriptome, ChIP-chip)
- 10.4 Repression of target genes by CcpA
- 10.5 Positive regulation of gene expression by CcpA
- 10.6 Control of CcpA activity
- 10.7 CcpA-DNA interaction
- 10.8 Functional analysis of CcpA
- 10.9 Structural analyses
Categories containing this gene/protein
- see also: glutamate metabolism
This gene is a member of the following regulons
The CcpA regulon
The gene
Basic information
- Locus tag: BSU29740
Phenotypes of a mutant
Loss of carbon catabolite repression. Loss of PTS-dependent sugar transport due to excessive phosphorylation of HPr by HprK. The mutant is unable to grow on a minimal medium with glucose and ammonium as the only sources of carbon and nitrogen, respectively.
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: transcriptional regulator of carbon catabolite repression (CCR)
- Protein family: LacI family
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- HTH LacI-type Domain (1 – 58)
- DNA binding Domain (6 – 25)
- Modification:
- Effectors of protein activity:glucose-6-phosphate, fructose-1,6-bisphosphate Pubmed
Database entries
- Structure:
- 2JCG (Apoprotein from Bacillus megaterium)
- CcpA-Crh-DNA-complex NCBI
- complex with P-Ser-HPr and sulphate ions NCBI
- 3OQM (complex of B. subtilis CcpA with P-Ser-HPr and the ackA operator site)
- 3OQN (complex of B. subtilis CcpA with P-Ser-HPr and the gntR operator site)
- 3OQO (complex of B. subtilis CcpA with P-Ser-HPr and a optimal synthetic operator site)
- UniProt: P25144
- KEGG entry: [3]
Additional information
Expression and regulation
- Sigma factor:
- Regulation: constitutively expressed PubMed
- Additional information: there are about 3.000 molecules of CcpA per cell PubMed, this corresponds to a concentration of 3 myM (according to PubMed)
Biological materials
- Mutant:
- QB5407 (spc), available in Jörg Stülke's lab
- GP302 (erm), available in Jörg Stülke's lab
- GP300 (an in frame deletion of ccpA), available in Jörg Stülke's lab
- WH649 (aphA3), available in Gerald Seidel's lab
- Expression vector:
- pGP643 (N-terminal Strep-tag, purification from B. subtilis, for SPINE, in pGP380), available in Jörg Stülke's lab
- pWH940 (C-terminal Strep-tag, purification from B. subtilis, for SPINE, in pGP382), available in Gerald Seidel's lab
- Strep-tag construct: GP1303 ccpA-Strep (spc) in native locus, based on (pGP1389), available in Jörg Stülke's lab
- lacZ fusion:
- GFP fusion:
- Antibody: available in Gerald Seidel's and in Jörg Stülke's lab
Labs working on this gene/protein
- Gerald Seidel, Erlangen University, Germany Homepage
- Richard Brennan, Houston, Texas, USA Homepage
- Milton H. Saier, University of California at San Diego, USA Homepage
- Yasutaro Fujita, University of Fukuyama, Japan
- Jörg Stülke, University of Göttingen, Germany Homepage
- Oscar Kuipers, University of Groningen, The Netherlands Homepage
Your additional remarks
References
Reviews
General and physiological studies
Global analyses (proteome, transcriptome, ChIP-chip)
Repression of target genes by CcpA
Positive regulation of gene expression by CcpA
Control of CcpA activity
CcpA-DNA interaction
Maria A Schumacher, Mareen Sprehe, Maike Bartholomae, Wolfgang Hillen, Richard G Brennan
Structures of carbon catabolite protein A-(HPr-Ser46-P) bound to diverse catabolite response element sites reveal the basis for high-affinity binding to degenerate DNA operators.
Nucleic Acids Res: 2011, 39(7);2931-42
[PubMed:21106498]
[WorldCat.org]
[DOI]
(I p)
Gerald Seidel, Marco Diel, Norbert Fuchsbauer, Wolfgang Hillen
Quantitative interdependence of coeffectors, CcpA and cre in carbon catabolite regulation of Bacillus subtilis.
FEBS J: 2005, 272(10);2566-77
[PubMed:15885105]
[WorldCat.org]
[DOI]
(P p)
Y Miwa, A Nakata, A Ogiwara, M Yamamoto, Y Fujita
Evaluation and characterization of catabolite-responsive elements (cre) of Bacillus subtilis.
Nucleic Acids Res: 2000, 28(5);1206-10
[PubMed:10666464]
[WorldCat.org]
[DOI]
(I p)
J H Kim, G H Chambliss
Contacts between Bacillus subtilis catabolite regulatory protein CcpA and amyO target site.
Nucleic Acids Res: 1997, 25(17);3490-6
[PubMed:9254709]
[WorldCat.org]
[DOI]
(P p)
Y Fujita, Y Miwa, A Galinier, J Deutscher
Specific recognition of the Bacillus subtilis gnt cis-acting catabolite-responsive element by a protein complex formed between CcpA and seryl-phosphorylated HPr.
Mol Microbiol: 1995, 17(5);953-60
[PubMed:8596444]
[WorldCat.org]
[DOI]
(P p)
J H Kim, Z T Guvener, J Y Cho, K C Chung, G H Chambliss
Specificity of DNA binding activity of the Bacillus subtilis catabolite control protein CcpA.
J Bacteriol: 1995, 177(17);5129-34
[PubMed:7665492]
[WorldCat.org]
[DOI]
(P p)
Functional analysis of CcpA
Structural analyses