Difference between revisions of "McsB"
(→Original Publications) |
|||
| Line 136: | Line 136: | ||
* '''Mutant:''' | * '''Mutant:''' | ||
| − | **''mcsB::aphA3'' availbale from the Gerth lab | + | ** ''mcsB::aphA3'' availbale from the Gerth lab |
| − | **mcsBC167S::spec available from the Gerth lab | + | ** mcsBC167S::spec available from the Gerth lab |
| − | **GP1457 (''mcsB''::''aphA3''), available in [[Stülke]] lab | + | ** GP1457 (''mcsB''::''aphA3''), available in [[Jörg Stülke]]'s lab |
| − | **BP69 (spc), available in [[Fabian Commichau]]'s lab | + | ** BP69 (spc), available in [[Fabian Commichau]]'s lab |
| + | |||
* '''Expression vector:''' for expression, purification in E. coli with N-terminal His-tag, pRSETA available in Gerth lab | * '''Expression vector:''' for expression, purification in E. coli with N-terminal His-tag, pRSETA available in Gerth lab | ||
| Line 154: | Line 155: | ||
* [[Ulf Gerth]], Greifswald, Germany | * [[Ulf Gerth]], Greifswald, Germany | ||
| − | * [[ | + | * [[Fabian Commichau]] Göttingen, Germany |
=Your additional remarks= | =Your additional remarks= | ||
| Line 164: | Line 165: | ||
<pubmed> 23375660 19609260</pubmed> | <pubmed> 23375660 19609260</pubmed> | ||
==Original Publications== | ==Original Publications== | ||
| − | <pubmed>17380125,16163393,19498169 11179229,9987115, 8793870, 16497325,19226326, 9987115, 11544224, 20852588 21622759 22517742 24825175 24263382</pubmed> | + | <pubmed>17380125,16163393,19498169 11179229,9987115, 8793870, 16497325,19226326, 9987115, 11544224, 20852588 21622759 22517742 24825175 24263382 25610436</pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 09:07, 23 January 2015
- Description: protein arginine kinase, adaptor protein, modulator of CtsR-dependent repression
| Gene name | mcsB |
| Synonyms | yacI |
| Essential | no |
| Product | protein arginine kinase |
| Function | control of CtsR activity |
| Gene expression levels in SubtiExpress: mcsB | |
| Interactions involving this protein in SubtInteract: McsB | |
| Metabolic function and regulation of this protein in SubtiPathways: mcsB | |
| MW, pI | 40 kDa, 5.068 |
| Gene length, protein length | 1089 bp, 363 aa |
| Immediate neighbours | mcsA, clpC |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 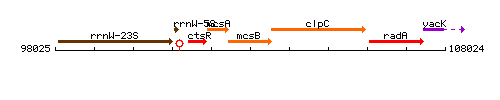
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed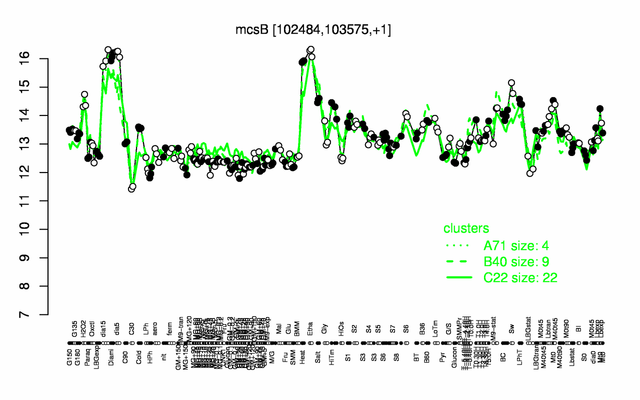
| |
Contents
Categories containing this gene/protein
protein modification, transcription factors and their control, sporulation proteins, general stress proteins (controlled by SigB), heat shock proteins
This gene is a member of the following regulons
CtsR regulon, SigB regulon, SigF regulon
The gene
Basic information
- Locus tag: BSU00850
Phenotypes of a mutant
Database entries
- BsubCyc: BSU00850
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: targets non-functional CtsR for degradation by ClpP/ClpC PubMed
- Protein family: ATP:guanido phosphotransferase family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Modification:
- Effectors of protein activity:
Database entries
- BsubCyc: BSU00850
- Structure:
- UniProt: P37570
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulation:
- Additional information:
- number of protein molecules per cell (minimal medium with glucose and ammonium): 142 PubMed
Biological materials
- Mutant:
- mcsB::aphA3 availbale from the Gerth lab
- mcsBC167S::spec available from the Gerth lab
- GP1457 (mcsB::aphA3), available in Jörg Stülke's lab
- BP69 (spc), available in Fabian Commichau's lab
- Expression vector: for expression, purification in E. coli with N-terminal His-tag, pRSETA available in Gerth lab
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody: available in Gerth lab
Labs working on this gene/protein
- Ulf Gerth, Greifswald, Germany
- Fabian Commichau Göttingen, Germany
Your additional remarks
References
Reviews
Original Publications
Lorena Stannek, Katrin Gunka, Rachel A Care, Ulf Gerth, Fabian M Commichau
Factors that mediate and prevent degradation of the inactive and unstable GudB protein in Bacillus subtilis.
Front Microbiol: 2014, 5;758
[PubMed:25610436]
[WorldCat.org]
[DOI]
(P e)
Débora Broch Trentini, Jakob Fuhrmann, Karl Mechtler, Tim Clausen
Chasing Phosphoarginine Proteins: Development of a Selective Enrichment Method Using a Phosphatase Trap.
Mol Cell Proteomics: 2014, 13(8);1953-64
[PubMed:24825175]
[WorldCat.org]
[DOI]
(I p)
Andreas Schmidt, Débora Broch Trentini, Silvia Spiess, Jakob Fuhrmann, Gustav Ammerer, Karl Mechtler, Tim Clausen
Quantitative phosphoproteomics reveals the role of protein arginine phosphorylation in the bacterial stress response.
Mol Cell Proteomics: 2014, 13(2);537-50
[PubMed:24263382]
[WorldCat.org]
[DOI]
(I p)
Alexander K W Elsholz, Kürsad Turgay, Stephan Michalik, Bernd Hessling, Katrin Gronau, Dan Oertel, Ulrike Mäder, Jörg Bernhardt, Dörte Becher, Michael Hecker, Ulf Gerth
Global impact of protein arginine phosphorylation on the physiology of Bacillus subtilis.
Proc Natl Acad Sci U S A: 2012, 109(19);7451-6
[PubMed:22517742]
[WorldCat.org]
[DOI]
(I p)
A K W Elsholz, K Hempel, S Michalik, K Gronau, D Becher, M Hecker, U Gerth
Activity control of the ClpC adaptor McsB in Bacillus subtilis.
J Bacteriol: 2011, 193(15);3887-93
[PubMed:21622759]
[WorldCat.org]
[DOI]
(I p)
Alexander K W Elsholz, Stephan Michalik, Daniela Zühlke, Michael Hecker, Ulf Gerth
CtsR, the Gram-positive master regulator of protein quality control, feels the heat.
EMBO J: 2010, 29(21);3621-9
[PubMed:20852588]
[WorldCat.org]
[DOI]
(I p)
Jakob Fuhrmann, Andreas Schmidt, Silvia Spiess, Anita Lehner, Kürsad Turgay, Karl Mechtler, Emmanuelle Charpentier, Tim Clausen
McsB is a protein arginine kinase that phosphorylates and inhibits the heat-shock regulator CtsR.
Science: 2009, 324(5932);1323-7
[PubMed:19498169]
[WorldCat.org]
[DOI]
(I p)
Jeanette Hahn, Naomi Kramer, Kenneth Briley, David Dubnau
McsA and B mediate the delocalization of competence proteins from the cell poles of Bacillus subtilis.
Mol Microbiol: 2009, 72(1);202-15
[PubMed:19226326]
[WorldCat.org]
[DOI]
(I p)
Janine Kirstein, David A Dougan, Ulf Gerth, Michael Hecker, Kürşad Turgay
The tyrosine kinase McsB is a regulated adaptor protein for ClpCP.
EMBO J: 2007, 26(8);2061-70
[PubMed:17380125]
[WorldCat.org]
[DOI]
(P p)
Stephanie T Wang, Barbara Setlow, Erin M Conlon, Jessica L Lyon, Daisuke Imamura, Tsutomu Sato, Peter Setlow, Richard Losick, Patrick Eichenberger
The forespore line of gene expression in Bacillus subtilis.
J Mol Biol: 2006, 358(1);16-37
[PubMed:16497325]
[WorldCat.org]
[DOI]
(P p)
Janine Kirstein, Daniela Zühlke, Ulf Gerth, Kürşad Turgay, Michael Hecker
A tyrosine kinase and its activator control the activity of the CtsR heat shock repressor in B. subtilis.
EMBO J: 2005, 24(19);3435-45
[PubMed:16163393]
[WorldCat.org]
[DOI]
(P p)
A Petersohn, M Brigulla, S Haas, J D Hoheisel, U Völker, M Hecker
Global analysis of the general stress response of Bacillus subtilis.
J Bacteriol: 2001, 183(19);5617-31
[PubMed:11544224]
[WorldCat.org]
[DOI]
(P p)
E Krüger, D Zühlke, E Witt, H Ludwig, M Hecker
Clp-mediated proteolysis in Gram-positive bacteria is autoregulated by the stability of a repressor.
EMBO J: 2001, 20(4);852-63
[PubMed:11179229]
[WorldCat.org]
[DOI]
(P p)
I Derré, G Rapoport, T Msadek
CtsR, a novel regulator of stress and heat shock response, controls clp and molecular chaperone gene expression in gram-positive bacteria.
Mol Microbiol: 1999, 31(1);117-31
[PubMed:9987115]
[WorldCat.org]
[DOI]
(P p)
E Krüger, T Msadek, M Hecker
Alternate promoters direct stress-induced transcription of the Bacillus subtilis clpC operon.
Mol Microbiol: 1996, 20(4);713-23
[PubMed:8793870]
[WorldCat.org]
[DOI]
(P p)